The equilibrium constant for the acid ionization of mercaptoethanol (HSCH2CH2OH ) is 1.91 10-10. HSCH2CH2OH(aq) 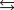 H-(aq) + SCH2CH2OH-(aq)
H-(aq) + SCH2CH2OH-(aq)
Which of the following is true regarding this equilibrium?
I.The reaction is product-favored.
II.The reaction is reactant-favored.
III.Equilibrium lies far to the right.
IV.Equilibrium lies far to the left.
A) I and III
B) I and IV
C) II and III
D) II and IV
E) None are true, as the concentrations of reactants and products are comparable.
Correct Answer:
Verified
Q33: Calculate K for the following reaction, provided
Q34: Equilibrium constants for gases can be expressed
Q35: Which expression corresponds to the equilibrium constant
Q36: The chemical equilibrium constant for the
Q37: An increase in the number of moles
Q39: The equilibrium constant for the formation
Q40: For the following reaction, Kp =
Q41: In the following reaction, which of the
Q42: Given the following reactions and associated
Q43: If the reaction quotient Q has a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents