Calculate K for the following reaction, provided the concentration versus time graph shown below. 3A  B + 2C
B + 2C 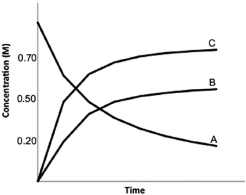
A) 2.0
B) 10
C) 22
D) 31
E) 44
Correct Answer:
Verified
Q28: Water can decompose at an elevated
Q29: Sulfur dioxide, gaseous water, and oxygen
Q30: For the following hypothetical equilibrium, what
Q31: Equilibrium constants can be expressed in terms
Q32: Coal (C) can be heated with steam
Q34: Equilibrium constants for gases can be expressed
Q35: Which expression corresponds to the equilibrium constant
Q36: The chemical equilibrium constant for the
Q37: An increase in the number of moles
Q38: The equilibrium constant for the acid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents