Sulfur dioxide, gaseous water, and oxygen gas react to prepare sulfuric acid in the following unbalanced equation. 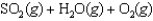
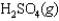 Under equilibrium conditions, the partial pressures of the components are PSO2 = 0.1 atm, PH2O = 0.05 atm, PO2 = 0.25 atm, PH2SO4 = 2.75 atm.
Under equilibrium conditions, the partial pressures of the components are PSO2 = 0.1 atm, PH2O = 0.05 atm, PO2 = 0.25 atm, PH2SO4 = 2.75 atm.
What is the value of the equilibrium constant, Kp, for the reaction?
A) 3.30 10-4
B) 5.00 10-4
C) 2.20 103
D) 3.03 103
E) 1.21 106
Correct Answer:
Verified
Q24: Under what conditions are the values of
Q25: Two students measured an equilibrium constant for
Q26: The equilibrium constant for the formation of
Q27: For the following reaction, Kc =
Q28: Water can decompose at an elevated
Q30: For the following hypothetical equilibrium, what
Q31: Equilibrium constants can be expressed in terms
Q32: Coal (C) can be heated with steam
Q33: Calculate K for the following reaction, provided
Q34: Equilibrium constants for gases can be expressed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents