The equilibrium constant for the formation of hydrogen iodide from hydrogen and iodine is 45.0 at a certain temperature. H2(g) + I2(s) 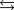 2 HI(g)
2 HI(g)
Which of the following is true regarding this equilibrium?
I.The reaction is product-favored.
II.The reaction is reactant-favored.
III.Equilibrium lies to the right.
IV.Equilibrium lies to the left.
A) I and III
B) I and IV
C) II and III
D) II and IV
E) None are true, as the concentrations of reactants and products are essentially the same.
Correct Answer:
Verified
Q21: Calculate K for the following reaction, provided
Q22: An equilibrium that strongly favors products has
Q23: A decrease in the number of moles
Q24: Under what conditions are the values of
Q25: Two students measured an equilibrium constant for
Q27: For the following reaction, Kc =
Q28: Water can decompose at an elevated
Q29: Sulfur dioxide, gaseous water, and oxygen
Q30: For the following hypothetical equilibrium, what
Q31: Equilibrium constants can be expressed in terms
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents