Calculate K for the following reaction, provided the concentration versus time graph shown below. 2A  2B + 3C
2B + 3C 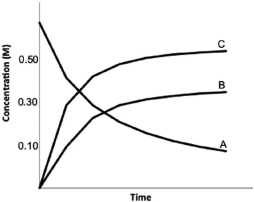
A) 0.50
B) 0.68
C) 1.1
D) 1.5
E) 2.8
Correct Answer:
Verified
Q16: Which of the following is true for
Q17: Which of A-D is equal in an
Q18: In a reversible reaction A 
Q19: Consider the equilibrium A + B
Q20: At a high temperature, carbon dioxide decomposes
Q22: An equilibrium that strongly favors products has
Q23: A decrease in the number of moles
Q24: Under what conditions are the values of
Q25: Two students measured an equilibrium constant for
Q26: The equilibrium constant for the formation of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents