At a high temperature, carbon dioxide decomposes to produce carbon monoxide and oxygen. Which expression corresponds to the equilibrium constant for the reaction written as 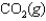
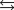
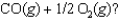
A) 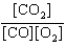
B) 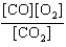
C) 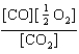
D) 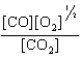
E) 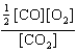
Correct Answer:
Verified
Q15: Which of the following is true for
Q16: Which of the following is true for
Q17: Which of A-D is equal in an
Q18: In a reversible reaction A 
Q19: Consider the equilibrium A + B
Q21: Calculate K for the following reaction, provided
Q22: An equilibrium that strongly favors products has
Q23: A decrease in the number of moles
Q24: Under what conditions are the values of
Q25: Two students measured an equilibrium constant for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents