In a reversible reaction A 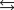 B at a particular temperature, the rate constant for the reverse reaction is 4 times the rate constant for the forward reaction. What is the value of the equilibrium constant at that temperature?
B at a particular temperature, the rate constant for the reverse reaction is 4 times the rate constant for the forward reaction. What is the value of the equilibrium constant at that temperature?
A) Can't tell, need more information.
B) 0.25
C) 4
D) 16
E) 0.5
Correct Answer:
Verified
Q13: Which of the following is true for
Q14: For an equilibrium reaction with K
Q15: Which of the following is true for
Q16: Which of the following is true for
Q17: Which of A-D is equal in an
Q19: Consider the equilibrium A + B
Q20: At a high temperature, carbon dioxide decomposes
Q21: Calculate K for the following reaction, provided
Q22: An equilibrium that strongly favors products has
Q23: A decrease in the number of moles
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents