The reaction of nitrogen gas with oxygen gas, shown here, has a Kp value of 0.25. If a closed vessel was charged with the two reactants, each at an initial partial pressure of 0.250 atm, what would be the equilibrium partial pressure of NO(g) ?
N2(g) + O2(g) 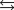 2NO(g)
2NO(g)
A) 0.100 atm
B) 0.125 atm
C) 0.166 atm
D) 0.228 atm
E) 0.351 atm
Correct Answer:
Verified
Q83: To solve an equation of the form
Q84: When can an x be ignored in
Q85: The reaction of bromine gas with chlorine
Q86: Hydrofluoric acid is used in the preparation
Q87: The equilibrium constant for the reaction below
Q89: A student determined the equilibrium concentration
Q90: Using the Reaction, Initial, Change, Equilibrium (RICE)
Q91: Calculate K for the following reaction, provided
Q92: For the reaction of hydrogen and
Q93: The equilibrium constants for the two reactions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents