Calculate K for the following reaction, provided the concentration versus time graph shown below.
2A  2B + 3C
2B + 3C 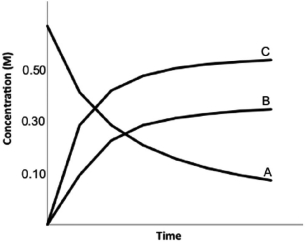
Correct Answer:
Verified
Q86: Hydrofluoric acid is used in the preparation
Q87: The equilibrium constant for the reaction below
Q88: The reaction of nitrogen gas with oxygen
Q89: A student determined the equilibrium concentration
Q90: Using the Reaction, Initial, Change, Equilibrium (RICE)
Q92: For the reaction of hydrogen and
Q93: The equilibrium constants for the two reactions
Q94: The gas-phase equilibrium of the oxidation
Q95: A 10.0 L flask at 500 K
Q96: The reaction of bromine gas with chlorine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents