The reaction of bromine gas with chlorine gas, shown here, has a Kp value of 7.20. If a closed vessel was charged with the two reactants, each at an initial partial pressure of 0.500 atm, and the product also at 0.500 atm, what would be the equilibrium partial pressure of BrCl(g) ?
Br2(g) + Cl2(g) 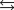 2BrCl(g)
2BrCl(g)
A) 0.500 atm
B) 0.680 atm
C) 0.859 atm
D) 0.029 atm
E) 0.987 atm
Correct Answer:
Verified
Q91: Calculate K for the following reaction, provided
Q92: For the reaction of hydrogen and
Q93: The equilibrium constants for the two reactions
Q94: The gas-phase equilibrium of the oxidation
Q95: A 10.0 L flask at 500 K
Q97: Write an expression for the equilibrium constant
Q98: Chemical equilibrium arises from the _ of
Q99: For the reaction of hydrogen and
Q100: The reaction of bromine gas with chlorine
Q101: If Q for a reaction is greater
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents