One reaction that contributes to acid rain is the conversion of dinitrogen pentoxide to nitric acid upon reaction with water. How could the rate of this reaction be expressed correctly in terms of the rate at which the concentration of a reactant or product changes?
N2O5(g) + H2O(g)  2HNO3(g)
2HNO3(g)
A) Rate = 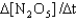
B) Rate =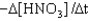
C) Rate = 
D) Rate = 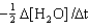
E) Rate= 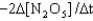
Correct Answer:
Verified
Q13: At what time of day are ozone
Q14: The difference between an average rate and
Q15: For the reaction 2A + 3B
Q16: Smog created by the interaction of sunlight
Q17: The device in automobiles that has decreased
Q19: One reaction that occurs in an automobile
Q20: One reaction that occurs in an automobile
Q21: From the following concentration versus time plot
Q22: In the combustion of methane, 
Q23: Which of the following is a possible
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents