One reaction that occurs in an automobile catalytic converter is the conversion of nitrogen monoxide to nitrogen and oxygen. How is the rate of this reaction related to the rate at which the concentration of a reactant or product changes?
2NO(g) →N2(g) +O2(g)
I. Rate = 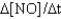
II. Rate = 
III. Rate = 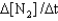
IV. Rate = 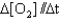
A) I only
B) II only
C) III and IV only
D) I, III, and IV only
E) II, III, and IV only
Correct Answer:
Verified
Q15: For the reaction 2A + 3B
Q16: Smog created by the interaction of sunlight
Q17: The device in automobiles that has decreased
Q18: One reaction that contributes to acid rain
Q19: One reaction that occurs in an automobile
Q21: From the following concentration versus time plot
Q22: In the combustion of methane, 
Q23: Which of the following is a possible
Q24: A scientist conducts an experiment to determine
Q25: The following graph shows the kinetics curves
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents