Using the reaction shown below, which statement is correct? 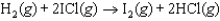
A) The concentration of all compounds are changing by the same amount at all times.
B) The HCl concentration increases at the same rate as the concentration of iodine.
C) The concentration of hydrogen decreases at the same rate as the concentration of ICl.
D) The concentration of HCl increases twice as fast as the concentration of iodine.
E) None of these statements is correct.
Correct Answer:
Verified
Q34: Assuming that each of the following graphs
Q35: A scientist conducts an experiment to determine
Q36: If the rate of formation of dinitrogen
Q37: If the rate of formation of nitrogen
Q38: If the rate of formation of ammonia
Q40: If the rate of formation of ammonia
Q41: The rate law of a particular reaction
Q42: For the rate law Rate = k[A][B]1/2,
Q43: The half-life (t1/2) of a first-order reaction
Q44: In a rate law, the partial orders
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents