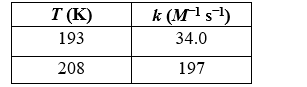
Chlorine dioxide formed from chlorofluorocarbons was found to catalyze the decomposition of atmospheric ozone. In laboratory experiments, the rate of the catalyzed reaction was measured at different temperatures with the following results. What is the activation energy of this reaction?
A) 2.81 kJ/mol
B) 3.70 kJ/mol
C) 14.6 kJ/mol
D) 22.9 kJ/mol
E) 39.1 kJ/mol
Correct Answer:
Verified
Q82: Given the following data for the reaction
Q83: The rate at which popcorn pops was
Q84: The following figure shows Arrhenius plots for
Q85: The high temperatures in an automobile engine
Q86: The energy profiles for four different reactions
Q88: The energy profiles for four different reactions
Q89: Given the following data for the reaction
Q90: Which statement A-D regarding the reaction described
Q91: The following figure shows Arrhenius plots for
Q92: The rate of popcorn popping at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents