Multiple Choice
The mechanism for the first-order reaction 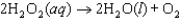
in the presence of I=-=(aq) is proposed to be:
Step 1: 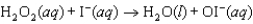 (slow)
(slow)
Step 2: 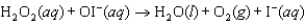 (fast)
(fast)
Identify the catalyst in the reaction.
A) H2O2
B) OI-
C) I-
D) H2O
E) O2
Correct Answer:
Verified
Related Questions
Q135: For the reaction A 
Q136: The steps in a reaction mechanism are
Q137: The order in which ozone, nitrogen monoxide,
Q138: The photochemical decomposition of chlorofluorocarbons 
Q139: For the rate law Rate = k[A]1/2[B],
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents