Identify the order of each of the following reactions, based on the supplied graphs. 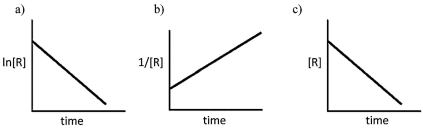
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q139: For the rate law Rate = k[A]1/2[B],
Q140: The mechanism for the first-order reaction
Q141: The second-order reaction A Q142: In a first-order reaction, the initial concentration Q143: In a(n) _ step of a reaction Q145: In a catalyzed reaction, the activation energy Q146: For the following energy profile: Q147: The linear form of the Arrhenius equation Q148: In a catalyzed reaction, the _ of Q149: The linear form of the _ is![]()
a) identify any
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents