For the following energy profile:
a) identify any reaction intermediates,
b) identify any transition states,
c) state the number of steps,
d) state whether the reaction is endo- or exothermic,
e) indicate which step is rate determining. 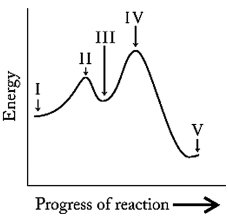
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q141: The second-order reaction A Q142: In a first-order reaction, the initial concentration Q143: In a(n) _ step of a reaction Q144: Identify the order of each of the Q145: In a catalyzed reaction, the activation energy Q147: The linear form of the Arrhenius equation Q148: In a catalyzed reaction, the _ of Q149: The linear form of the _ is Q150: The mechanism for the reaction Q151: For the rate law Rate =k[A][B]3/2, the![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents