The mechanism for the reaction 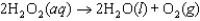
in the presence of I-(aq) is proposed to be:
Step 1: 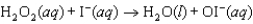 (slow)
(slow)
Step 2: 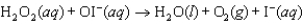 (fast)
(fast)
Identify a) any intermediates, b) any catalysts, and c) the rate law for the reaction.
Correct Answer:
Verified
b...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q145: In a catalyzed reaction, the activation energy
Q146: For the following energy profile:
a) identify any
Q147: The linear form of the Arrhenius equation
Q148: In a catalyzed reaction, the _ of
Q149: The linear form of the _ is
Q151: For the rate law Rate =k[A][B]3/2, the
Q152: Chlorine dioxide (ClO2) is used as a
Q153: Given the following data for the
Q154: The rate of popcorn popping at different
Q155: N2O5 is used as a source of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents