Ion interaction energies are determined by Coulomb's law: 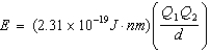 Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.
Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.
A) (-7.36 10-19 J)
B) (-7.36 10-22 J)
C) 7.36 10-19 J
D) 1.74 10-18 J
E) (-7.36 10-25 J)
Correct Answer:
Verified
Q15: Which of the following ranks the compounds
Q16: Which of the following must be true
Q17: Coulomb's law states that the energy of
Q18: Which statement A-D about ionic solids is
Q19: Which arrangement below orders the cations from
Q21: Which of the following requires the smallest
Q22: Which of the following ionic compounds would
Q23: Why would you expect or predict sodium
Q24: In the following Born-Haber cycle for the
Q25: In the following Born-Haber cycle for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents