In the following Born-Haber cycle for the formation of sodium chloride from its elements, which step (A-E) corresponds to the ionization energy of one of the reactants? 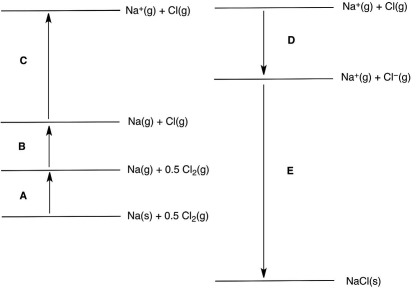
A) A
B) B
C) C
D) D
E) E
Correct Answer:
Verified
Q19: Which arrangement below orders the cations from
Q20: Ion interaction energies are determined by
Q21: Which of the following requires the smallest
Q22: Which of the following ionic compounds would
Q23: Why would you expect or predict sodium
Q25: In the following Born-Haber cycle for the
Q26: Which of the following will have the
Q27: Which of the following is not typically
Q28: Which of the following ionic compounds would
Q29: Which one of the ionic compounds below
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents