Consider the phase diagram for a substance shown here. The line between the solid and liquid phases has a positive slope because the solid phase is ________ than the liquid phase. 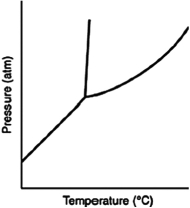
A) more dense
B) less dense
C) more massive
D) less massive
E) hotter
Correct Answer:
Verified
Q87: At the triple point of a substance,
Q88: Which of the following substances is
Q89: Consider the phase diagram for a substance
Q90: Freezing occurs in going from region _
Q91: The temperature at point b in the
Q93: On the phase diagram below, identify the
Q94: Condensation occurs in going from region _
Q95: The phase diagram for carbon dioxide is
Q96: The temperature at point a in the
Q97: On the phase diagram below, identify the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents