Consider the phase diagram for a substance such as the one shown here. If the line between the solid and liquid phases has a negative slope, then the solid phase is ________ than the liquid phase. 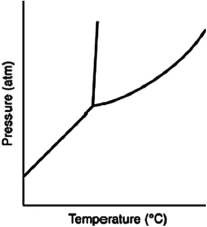
A) more dense
B) less dense
C) more massive
D) less massive
E) hotter
Correct Answer:
Verified
Q84: Which of the following substances has a
Q85: At the point marked with a dot
Q86: At the critical point, _
A)all the liquid
Q87: At the triple point of a substance,
Q88: Which of the following substances is
Q90: Freezing occurs in going from region _
Q91: The temperature at point b in the
Q92: Consider the phase diagram for a substance
Q93: On the phase diagram below, identify the
Q94: Condensation occurs in going from region _
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents