The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance has the lowest melting point? 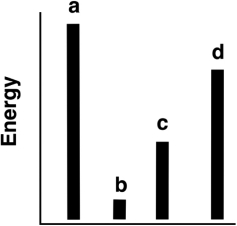
A) a
B) b
C) c
D) d
Correct Answer:
Verified
Q125: Given the van der Waals a constant
Q126: Given the van der Waals a constant
Q127: The boiling point of HBr is higher
Q128: Which one of the following substances would
Q129: Which of the following compounds would you
Q131: Under similar conditions, which of the following
Q132: Boiling points increase in the order HCl
Q133: The relative energies (strengths) of the intermolecular
Q134: Which compound do you predict has the
Q135: The relative energies (strengths) of the intermolecular
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents