Given the van der Waals a constant values for the following gases, which gas has the lowest boiling point?
A) CO2 a  3.64 L2 · atm/mol
3.64 L2 · atm/mol 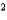
B) Ar a  1.36 L2 · atm/mol
1.36 L2 · atm/mol 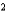
C) CCl4 a  19.75 L2 · atm/mol
19.75 L2 · atm/mol 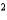
D) HBr a  4.51 L2 · atm/mol
4.51 L2 · atm/mol 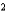
E) CO a  1.51 L2 · atm/mol
1.51 L2 · atm/mol 
Correct Answer:
Verified
Q121: The relative energies (strengths) of the intermolecular
Q122: In understanding why group 16 hydrides, other
Q123: What structural characteristics must a molecule have
Q124: Which of the following substances would you
Q125: Given the van der Waals a constant
Q127: The boiling point of HBr is higher
Q128: Which one of the following substances would
Q129: Which of the following compounds would you
Q130: The relative energies (strengths) of the intermolecular
Q131: Under similar conditions, which of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents