Multiple Choice
Which of the following is not a possible set of quantum numbers for an electron?
A) n  5,
5, 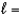 4, me
4, me
 2
2
B) n  3,
3, 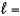 0, me
0, me  0
0
C) n  2,
2, 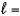 1, me
1, me  1
1
D) n  1,
1, 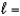 0, me
0, me  1
1
E) n  4,
4, 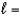 3, me
3, me  3
3
Correct Answer:
Verified
Related Questions
Q80: In quantum mechanics, an atomic orbital _
A)provides
Q81: Which orbital has the highest energy in
Q82: What are the principal and angular momentum
Q83: A coordinate system is defined with orthogonal
Q84: What is the orbital designation for an
Q86: Which of the following is a possible
Q87: Which of the following represents a p
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents