Which orbital has the highest energy in a multielectron atom? The quantum numbers are given as (n, 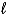 , ml) .
, ml) .
A) (3, 2, 0)
B) (4, 4, 0)
C) (3, 0, 0)
D) (4, 1, 1)
E) (4, 3, 1)
Correct Answer:
Verified
Q76: A shell is defined by _
A)the
Q77: Which statement about the quantum numbers that
Q78: According to de Broglie, if the circumference
Q79: The mathematical description of an electron as
Q80: In quantum mechanics, an atomic orbital _
A)provides
Q82: What are the principal and angular momentum
Q83: A coordinate system is defined with orthogonal
Q84: What is the orbital designation for an
Q85: Which of the following is not a
Q86: Which of the following is a possible
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents