Assuming that the distances between the two ions are the same in all cases, which of the following ion pairs has the greatest electrostatic potential energy (i.e., largest in magnitude) ?
A) 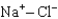
B) 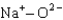
C) 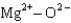
D) 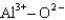
E) 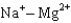
Correct Answer:
Verified
Q15: Which statement A-D about a state function
Q16: Thermochemistry is the study of how _
Q17: Energy that an object has by virtue
Q18: A diver jumps off a diving board.
Q19: Which statement A-D about the relationship between
Q21: Which arrow in the following diagrams represents
Q22: Internal energy is defined as _
A)the total
Q23: Which of the following bar charts shows
Q24: What is the change in internal
Q25: Which of the changes A-D will always
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents