Ammonium nitrate sometimes is used in cold packs because its enthalpy of solvation in water is +21 kJ/mol. What is the minimum amount of ammonium nitrate (80.05 g/mol) that is needed to cool a 1.0 L soft drink (d = 1.0 g/mL, specific heat 4.5 J g-1 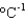 ) and its plastic bottle (heat capacity 50 J/K) from 30
) and its plastic bottle (heat capacity 50 J/K) from 30 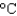 to 5
to 5 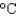 .
.
A) 540 g
B) 320 g
C) 110 g
D) 430 g
E) 250 g
Correct Answer:
Verified
Q58: An expanding gas does 175 kJ of
Q59: A heating curve for some substance is
Q60: Steam in a cylinder is compressed by
Q61: In an experiment, 30.0 g of
Q62: If 7.3 kJ of energy are required
Q64: Which of the following objects will cool
Q65: Given the following thermochemical equation detailing
Q66: When 1.14 g of octane (molar
Q67: A 15 g piece of iron
Q68: Given the following thermochemical equation detailing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents