Use the following information to determine the standard enthalpy change when 1 mol of PbO(s) is formed from lead metal and oxygen gas.
PbO(s) 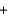 C(graphite)
C(graphite)  Pb(s)
Pb(s) 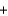 CO(g) H
CO(g) H  = 107 kJ
= 107 kJ
2C(graphite) 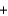 O2(g)
O2(g)  2CO(g) H = -222 kJ
2CO(g) H = -222 kJ
A) (- 436 kJ)
B) (+436 kJ)
C) (-218 kJ)
D) (-329 kJ)
E) (-115 kJ)
Correct Answer:
Verified
Q95: Determine the enthalpy of formation for
Q96: Use the following information to determine
Q97: Benzoic acid is used to determine
Q98: For which reaction below does the enthalpy
Q99: For which reaction below does the enthalpy
Q101: Which of the following hydrocarbons has the
Q102: Which of the following fuels has the
Q103: Ethanol (CH3CH2OH) has been suggested as
Q104: Describe the difference between potential energy and
Q105: Lightweight camping stoves typically use a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents