Which of the following fuels has the highest fuel value (kJ/g) ?
A) natural gas, CH4(g) , 16.0 g/mol, 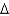 Hcomb
Hcomb  801 kJ/mol
801 kJ/mol
B) ethanol, CH3CH2OH(l) , 46.1 g/mol, 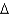 Hcomb
Hcomb  1,368 kJ/mol
1,368 kJ/mol
C) heating oil, C16H34(l) , 226 g/mol, 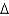 Hcomb
Hcomb  10,800 kJ/mol
10,800 kJ/mol
D) coal, C135H96O9NS(l) , 1906 g/mol, 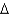 Hcomb
Hcomb  44,000 kJ/mol
44,000 kJ/mol
E) wood, C6H12O6(l) , 180 g/mol, 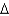 Hcomb
Hcomb  2,540 kJ/mol
2,540 kJ/mol
Correct Answer:
Verified
Q97: Benzoic acid is used to determine
Q98: For which reaction below does the enthalpy
Q99: For which reaction below does the enthalpy
Q100: Use the following information to determine
Q101: Which of the following hydrocarbons has the
Q103: Ethanol (CH3CH2OH) has been suggested as
Q104: Describe the difference between potential energy and
Q105: Lightweight camping stoves typically use a
Q106: Which of the following substances will release
Q107: Fuel value is _
A)the cost of energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents