Which of the following fuels has the lowest fuel value (kJ/g) ?
A) natural gas, CH4(g) , 16.0 g/mol, 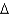 Hcomb
Hcomb  801 kJ/mol
801 kJ/mol
B) ethanol, CH3CH2OH(l) , 46.1 g/mol, 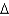 Hcomb
Hcomb  1,368 kJ/mol
1,368 kJ/mol
C) heating oil, C16H34(l) , 226 g/mol, 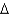 Hcomb
Hcomb  10,800 kJ/mol
10,800 kJ/mol
D) coal, C135H96O9NS(s) , 1906 g/mol, 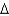 Hcomb
Hcomb  44,000 kJ/mol
44,000 kJ/mol
E) wood, C6H12O6(s) , 180 g/mol, 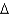 Hcomb
Hcomb  2,540 kJ/mol
2,540 kJ/mol
Correct Answer:
Verified
Q115: In a Reuters news report dated February
Q116: Ethanol (CH3CH2OH) has been suggested as
Q117: Which of the following hydrocarbons has the
Q118: Fuel density is _
A)the cost of energy
Q119: Ethanol (CH3CH2OH) has been suggested as
Q121: Benzoic acid is used to determine
Q122: Given the standard enthalpies of formation
Q123: The basal metabolic rate (BMR) is the
Q124: Sports trainers use cold packs containing
Q125: The complete combustion of 2.500 g
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents