Given the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction.
2N2H4(g) 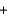 2NO2(g)
2NO2(g)  3N2(g)
3N2(g) 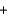 4H2O(g) Hrxn = ? kJ
4H2O(g) Hrxn = ? kJ
Substance H  in kJ/mol
in kJ/mol
N2H4(g) +95.4
NO2(g) +33.1
H2O(g) -241.8
Correct Answer:
Verified
Q117: Which of the following hydrocarbons has the
Q118: Fuel density is _
A)the cost of energy
Q119: Ethanol (CH3CH2OH) has been suggested as
Q120: Which of the following fuels has the
Q121: Benzoic acid is used to determine
Q123: The basal metabolic rate (BMR) is the
Q124: Sports trainers use cold packs containing
Q125: The complete combustion of 2.500 g
Q126: Determine the enthalpy for the following
Q127: To cool your 250 mL of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents