Determine the molar concentration of an aqueous solution of lead(II) nitrate solution that is 26 ppb (parts per billion) Pb(NO3) 2. Since the solution is very dilute, assume the density is that of water, 1.00 g/mL.
A) 7.85 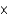
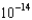 M
M
B) 2.60 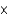
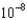 M
M
C) 4.26 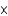
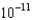 M
M
D) 7.85 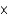
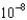 M
M
E) 7.85 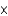
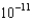 M
M
Correct Answer:
Verified
Q5: Lead is the least toxic metal ion
Q6: The National Primary Drinking Water Regulations limit
Q7: Assuming that the density of water is
Q8: What is the concentration, in g/L, of
Q9: What are the units of molar concentration,
Q11: A chemistry student attempted to make a
Q12: Molarity, M, is defined as _
A)moles of
Q13: A concentrated aqueous ammonia solution has a
Q14: Sodium fluoride (NaF) is added to drinking
Q15: A homogeneous mixture of two or more
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents