Sodium fluoride (NaF) is added to drinking water in some municipalities to protect teeth against cavities. The idea is to convert hydroxyapatite, Ca10(PO4) 6(OH) 2, into more stable fluorapatite, Ca10(PO4) 6F2. The molar mass of hydroxyapatite is 502 g/mol. What is the molarity of a 10.0 mg/L sodium fluoride solution?
A) 2.38 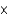
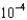 M
M
B) 1.99 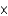
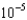 M
M
C) 2.63 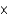
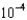 M
M
D) 1.19 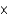
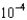 M
M
E) 0.263 M
Correct Answer:
Verified
Q9: What are the units of molar concentration,
Q10: Determine the molar concentration of an aqueous
Q11: A chemistry student attempted to make a
Q12: Molarity, M, is defined as _
A)moles of
Q13: A concentrated aqueous ammonia solution has a
Q15: A homogeneous mixture of two or more
Q16: Which one of the following mixtures is
Q17: Mercury is one of the most
Q18: If 120 g of NaOH were used
Q19: Which of the following cartoons depicts a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents