How many moles of magnesium nitrate are present in 75.0 mL of a 0.33 M solution?
A) 2.5 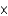
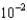 moles
moles
B) 25 moles
C) 4.4 moles
D) 2.3 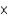
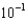 moles
moles
E) 4.4 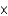
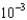 moles
moles
Correct Answer:
Verified
Q27: What volume of 3.0 M NaOH contains
Q28: What mass of potassium iodide (molar mass
Q29: How many moles of ammonium hydroxide are
Q30: Which contains more solute particles: a 0.10
Q31: How many grams of solid magnesium chloride,
Q33: Which picture best represents an atomic-level view
Q34: How many grams of magnesium chloride, MgCl2,
Q35: The World Health Organization recommends that the
Q36: What volume of 0.25 M hydrochloric acid
Q37: Hartmann's solution is used in intravenous therapy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents