Write the complete ionic equation for the reaction that takes place between a solution of lead(II) nitrate and sodium sulfate.
A) Pb(NO3) 2(aq)  Na2SO4(aq)
Na2SO4(aq)  PbSO4(s) +2NaNO3(aq)
PbSO4(s) +2NaNO3(aq)
B) Pb(NO3) 2(aq) 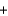 2Na+(aq)
2Na+(aq) 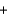 SO42-(aq)
SO42-(aq)  PbSO4(s)
PbSO4(s) 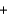 2Na+(aq)
2Na+(aq) 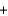 2NO3-(aq)
2NO3-(aq)
C) Pb2+(aq) 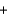 SO42-(aq)
SO42-(aq)  PbSO4(s)
PbSO4(s)
D) Pb2+(aq) 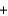 2NO3-(aq)
2NO3-(aq) 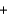 2Na+(aq)
2Na+(aq) 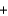 SO42-(aq)
SO42-(aq)  PbSO4(s)
PbSO4(s) 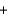 2Na+(aq)
2Na+(aq) 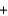 2NO3-(aq)
2NO3-(aq)
E) 2NO3-(aq) 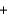 2Na+(aq)
2Na+(aq)  2NaNO3(aq)
2NaNO3(aq)
Correct Answer:
Verified
Q85: What mass of calcium hydroxide (molar mass
Q86: Which one of the following statements is
Q87: The oxidation number of carbon in carbon
Q88: The thermite reaction is used in welding
Q89: Which one of the following statements is
Q91: Ion-exchange resins can remove Mg2+ and Ca2+0
Q92: Silver salts are used in black-and-white photography.
Q93: What mass of lead(II) chloride is produced
Q94: What mass of barium sulfate (molar mass
Q95: What mass of silver chloride will be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents