The thermite reaction is used in welding applications and chemistry demonstrations because it releases a tremendous amount of energy. Which of the following statements is not correct regarding this reaction?
8Al 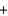 3Fe3O4
3Fe3O4  9Fe
9Fe 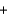 4Al2O3
4Al2O3
A) Aluminum is oxidized.
B) Iron is reduced.
C) The oxidation number of Al changes from 0 to +3.
D) Aluminum is the reducing agent.
E) Three electrons are transferred from each aluminum atom to each iron atom.
Correct Answer:
Verified
Q83: Chlorine gas easily gains two electrons to
Q84: Write the net ionic equation for the
Q85: What mass of calcium hydroxide (molar mass
Q86: Which one of the following statements is
Q87: The oxidation number of carbon in carbon
Q89: Which one of the following statements is
Q90: Write the complete ionic equation for the
Q91: Ion-exchange resins can remove Mg2+ and Ca2+0
Q92: Silver salts are used in black-and-white photography.
Q93: What mass of lead(II) chloride is produced
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents