Magnesium hydroxide is insoluble. Write the net ionic equation for the reaction that takes place between a solution of magnesium chloride and a solution of sodium hydroxide.
A) MgCl2(aq) 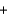 NaOH(aq)
NaOH(aq)  MgOH(s) + NaCl2(aq)
MgOH(s) + NaCl2(aq)
B) MgCl2(aq)  2NaOH(aq)
2NaOH(aq)  Mg(OH) 2(s) +2NaCl(aq)
Mg(OH) 2(s) +2NaCl(aq)
C) Mg2+(aq) 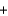 2OH-(aq)
2OH-(aq)  Mg(OH) 2(s)
Mg(OH) 2(s)
D) Mg2+(aq) 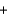 2Cl- (aq)
2Cl- (aq) 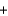 2Na+(aq)
2Na+(aq) 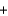 2OH-(aq)
2OH-(aq)  Mg(OH) 2(s)
Mg(OH) 2(s) 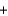 2NaCl(s)
2NaCl(s)
E) Mg2+(aq) 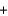 OH-(aq)
OH-(aq)  MgOH(s)
MgOH(s)
Correct Answer:
Verified
Q95: What mass of silver chloride will be
Q96: A reaction that takes place between dissolved
Q97: What is the oxidation number of S
Q98: Sodium metal easily loses an electron to
Q99: Identify the reducing agent in the following
Q101: In which one of the following compounds
Q102: Nitrogen-fixing bacteria and plants are capable of
Q103: Magnesium metal burns brightly in the air
Q104: Lead sinkers used for saltwater fishing do
Q105: Tube worms that survive near geothermal vents
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents