A reaction that takes place between dissolved barium nitrate and dissolved sodium sulfate results in formation of solid barium sulfate. Which equation describes this reaction?
A) Ba(NO3) 2(aq) 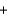 Na2SO4(aq)
Na2SO4(aq)  BaSO4(s)
BaSO4(s) 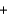 2NaNO3(aq)
2NaNO3(aq)
B) BaNO3(aq) 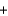 NaSO4(aq)
NaSO4(aq)  BaSO4(s)
BaSO4(s) 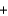 NaNO3(aq)
NaNO3(aq)
C) 2Ba(NO3) (aq) 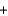 Na2SO4(aq)
Na2SO4(aq)  Ba2SO4(s)
Ba2SO4(s) 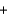 2NaNO3(aq)
2NaNO3(aq)
D) Ba(NO3) 2(aq) 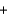 2NaSO4(aq)
2NaSO4(aq)  Ba(SO4) 2(s)
Ba(SO4) 2(s) 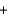 2NaNO3(aq)
2NaNO3(aq)
E) Ba(NO3) 2(aq) 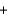 Na2SO4(aq)
Na2SO4(aq)  BaSO4(aq)
BaSO4(aq) 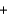 2NaNO3(s)
2NaNO3(s)
Correct Answer:
Verified
Q91: Ion-exchange resins can remove Mg2+ and Ca2+0
Q92: Silver salts are used in black-and-white photography.
Q93: What mass of lead(II) chloride is produced
Q94: What mass of barium sulfate (molar mass
Q95: What mass of silver chloride will be
Q97: What is the oxidation number of S
Q98: Sodium metal easily loses an electron to
Q99: Identify the reducing agent in the following
Q100: Magnesium hydroxide is insoluble. Write the net
Q101: In which one of the following compounds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents