Which of the following statements is not correct regarding the following redox reaction?
MnO2 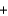 4HCl
4HCl  MnCl2
MnCl2 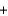 Cl2
Cl2
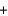 2H2O
2H2O
A) Manganese(IV) oxide is the oxidizing agent.
B) Chloride is oxidized.
C) The oxidation number of Mn changes from +4 to +2.
D) The oxidation number of Cl changes from -1 to 0.
E) One electron is transferred from manganese to each chloride.
Correct Answer:
Verified
Q76: Which one of the following water solubility
Q77: Water-soluble toxic chromium compounds are waste products
Q78: Ammonia (NH3) is a weak base that
Q79: In carrying out a titration of a
Q80: In carrying out a titration of a
Q82: Identify the oxidizing agent in the following
Q83: Chlorine gas easily gains two electrons to
Q84: Write the net ionic equation for the
Q85: What mass of calcium hydroxide (molar mass
Q86: Which one of the following statements is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents