Identify the oxidizing agent in the following reaction.
2Hg22+(aq) 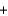 Sn2+(aq)
Sn2+(aq)  2Hg(l )
2Hg(l ) 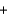 Sn4+(aq)
Sn4+(aq)
A) Hg(l)
B) Sn4+
C) Sn2+
D) Hg22+(aq)
E) This is not an example of an oxidation-reduction reaction.
Correct Answer:
Verified
Q77: Water-soluble toxic chromium compounds are waste products
Q78: Ammonia (NH3) is a weak base that
Q79: In carrying out a titration of a
Q80: In carrying out a titration of a
Q81: Which of the following statements is not
Q83: Chlorine gas easily gains two electrons to
Q84: Write the net ionic equation for the
Q85: What mass of calcium hydroxide (molar mass
Q86: Which one of the following statements is
Q87: The oxidation number of carbon in carbon
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents