Which statement A-D regarding the terms mole and molar mass is not correct?
A) A mole is defined as the number of particles in exactly 12 g of carbon-12.
B) A mole of oxygen gas contains 6.022 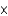 1023 molecules.
1023 molecules.
C) Two moles of oxygen atoms can be obtained by decomposing one mole of carbon dioxide.
D) To obtain the molar mass in grams (g/mol) from the atomic mass in atomic mass units(u) , multiply by 6.022 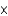 1023/mol and divide by 6.022
1023/mol and divide by 6.022 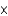 1023 u/g.
1023 u/g.
E) Statements A-D all are correct.
Correct Answer:
Verified
Q11: A sample of water (H2O) contains 1.81
Q12: Which of the following contains the largest
Q13: How many chlorine atoms are there in
Q14: Which statement about the combustion of propane
Q15: How many atoms of chlorine are there
Q17: Which substance listed below contains the most
Q18: In one analysis, 1.34 Q19: How many gold atoms are there in Q20: A tiny speck (8.3 Q21: How many mL of hexadecane (C16H34) are![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents