A sample of water (H2O) contains 1.81 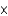 1024 molecules. How many total moles of atoms are there in this sample?
1024 molecules. How many total moles of atoms are there in this sample?
A) 1.00
B) 2.00
C) 3.00
D) 6.00
E) 9.00
Correct Answer:
Verified
Q6: A gallon of water has a mass
Q7: How many moles of ammonia are there
Q8: Which statement about the following chemical reaction
Q9: In one analysis, the density of ozone
Q10: For a standard airbag, 131 g of
Q12: Which of the following contains the largest
Q13: How many chlorine atoms are there in
Q14: Which statement about the combustion of propane
Q15: How many atoms of chlorine are there
Q16: Which statement A-D regarding the terms mole
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents