Carbon monoxide is used in refining iron ore to produce the metal. Assuming the reaction yield is 100%, how many grams of iron would be produced if 5.00 mol of carbon monoxide were exposed to 800 g iron(III) oxide?
Fe2O3(s) 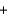 3CO(g)
3CO(g)  2Fe(s)
2Fe(s) 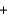 3CO2(g)
3CO2(g)
Correct Answer:
Verified
Q131: What is the difference in the chemistry
Q132: List three reasons why the actual yield
Q133: Write the balanced reaction equation for the
Q134: Balance the following chemical equation:
C2H3OCl 
Q135: Why is the term formula unit more
Q136: A range of organic molecules can undergo
Q137: Mesitylene is a liquid hydrocarbon. If 0.115
Q139: Write a definition of the phrase "stoichiometry
Q140: Methylheptenone (MHP) is used in producing lemon
Q141: Some indoor air purification systems work by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents