A range of organic molecules can undergo combustion. Pyridine (C5H5N) undergoes combustion in the unbalanced reaction shown below. Write the balanced equation, and find the percent yield for the reaction if 10.0 g of pyridine yields 18.1 g of carbon dioxide.
C5H5N 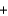 O2
O2
 H2O
H2O 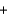 CO2
CO2 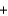 NO
NO
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q131: What is the difference in the chemistry
Q132: List three reasons why the actual yield
Q133: Write the balanced reaction equation for the
Q134: Balance the following chemical equation:
C2H3OCl 
Q135: Why is the term formula unit more
Q137: Mesitylene is a liquid hydrocarbon. If 0.115
Q138: Carbon monoxide is used in refining iron
Q139: Write a definition of the phrase "stoichiometry
Q140: Methylheptenone (MHP) is used in producing lemon
Q141: Some indoor air purification systems work by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents