Enzymes in plants convert nitrate into ammonia. The unbalanced half-reaction equation is given below. What is the stoichiometric coefficient of the electrons, e-, in the balanced reaction equation? NO3-(aq) + H2O( 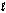 ) + e- NO2-(aq) + OH-(aq)
) + e- NO2-(aq) + OH-(aq)
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:
Verified
Q21: Plants convert nitrogen into ammonia. This
Q33: Iodine's role is very specific. It is
Q34: Enzymes in plants convert nitrate into
Q36: Rhenium-186 has medical applications. It decays
Q40: Plants convert nitrogen into ammonia. This
Q42: Iron accumulation in the human body has
Q43: Hearing aid batteries utilize a zinc/air
Q54: Chromium is an ultratrace metal in
Q65: Thallium-201 is used in cardiac imaging. It
Q65: Technetium can be produced in a nuclear
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents