Tooth Enamel Is Composed of the Mineral Hydroxyapatite Ca5 (PO4)3+(aq) + OH-(aq)
Ca5 (PO4)3(OH)(s) + H+(aq)
Tooth enamel is composed of the mineral hydroxyapatite. It is essentially insoluble in water with Ksp = 2.3 *10-59, but it reacts with weak acids in the mouth as described by one of the reaction equations below. You can determine the equilibrium constant, K, for this reaction from Ksp and Kw (the equilibrium constant for water autoionization, Kw = 1.0 * 10-14) . What is its value? Ca5 (PO4) 3(OH) (s) Ca5 (PO4) 3+(aq) + OH-(aq)
Ca5 (PO4) 3(OH) (s) + H+(aq) Ca5 (PO4) 3+(aq) + H2O( 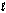 )
)
A) 2.3*10-73
B) 4.3 * 1045
C) 2.3 * 10-45
D) 1.1 * 10-14
E) 9.9 *10-15
Correct Answer:
Verified
Q3: What is the dominant role played by
Q16: Which ion is important in transmitting nerve
Q19: Which statement regarding mercury poisoning is not
Q44: The active site of an enzyme typically
Q68: Barium is a nonessential element in
Q69: Tooth enamel is composed of the
Q74: An enzyme catalyzes the decomposition of hydrogen
Q75: The cation BiO+ is found in some
Q77: The essential elements found in the human
Q79: A patient is injected with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents