An enzyme catalyzes the decomposition of hydrogen peroxide at 20°C. The activation energy for the uncatalyzed reaction is 75.3 kJ/mol. The activation energy for the catalyzed reaction is 29.3 kJ/mol. By what factor is the rate of the reaction increased by the enzyme? Use the Arrhenius equation, which is given below, to determine the ratio of rate constants, kcatalyzed/kuncatalyzed. (R = 8.315 J/mol K) 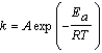
A) 1.6 *105
B) 1.6 *1011
C) 1.6 * 108
D) 7.6
E) 6100
Correct Answer:
Verified
Q16: Which ion is important in transmitting nerve
Q18: The ion found in chlorophyll is _
A)
Q69: Tooth enamel is composed of the
Q70: Tooth enamel is composed of the
Q75: The cation BiO+ is found in some
Q76: A radioactive isotope that emits the shortest
Q77: The essential elements found in the human
Q79: A patient is injected with a
Q80: Calcium is a major essential element in
Q90: Ions need to move in and out
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents