It is interesting to compare the energy produced by fusion to that produced by combustion. When carbon burns to produce carbon dioxide, 395 kJ/mol of energy is released. Approximately how much greater is the energy per mole of deuterium that is released in the following fusion process: 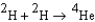 ? The atomic masses are 2.0104 g/mol for deuterium and 4.0026 g/mol for helium. Be sure to keep track of the units and get them correct; remember, 1 kJ = 1000 kg m2/s2.
? The atomic masses are 2.0104 g/mol for deuterium and 4.0026 g/mol for helium. Be sure to keep track of the units and get them correct; remember, 1 kJ = 1000 kg m2/s2.
A) 102
B) 104
C) 106
D) 108
E) 1010
Correct Answer:
Verified
Q28: Nitrogen-13 decays by positron emission to produce
Q72: A nerdy Scotch-drinking physicist, Nicola, suspected that
Q81: Write nuclear reaction equations to show how
Q100: The argon-40 method of radioisotope dating
Q122: In the initial sequence of thorium-232 decay,
Q137: Uranium-238 decays to lead-206 through a
Q142: Tritium (3H) is used in glowing "EXIT"
Q143: Bombarding atoms with alpha particles is a
Q148: Strontium-90 is most likely to decay by
Q161: When an atom of uranium-235 captures a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents