In a simple equilibrium A + B 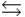 C, will there be any stress to the system if both B and C were added to the equilibrium system simultaneously and in the same amount?
C, will there be any stress to the system if both B and C were added to the equilibrium system simultaneously and in the same amount?
A) No, these two stresses will always cancel each other out.
B) No, these two stresses will cancel each other out unless the initial concentrations of B and C are also the same.
C) Yes, the same amount of A is also required for the stresses to cancel.
D) Yes, unless the initial concentrations of B and C are also the same.
E) More than two of the above statements are correct.
Correct Answer:
Verified
Q49: In the equation relating equilibrium to
Q53: The reaction of bromine gas with chlorine
Q53: What is the difference between
Q55: The reaction of bromine gas with chlorine
Q56: A reaction X + 2Y
Q57: When can an x be ignored in
Q59: Which of the following occurs when products
Q65: If the temperature of an endothermic reaction
Q65: Which of the following must be
Q76: Increasing the temperature of an exothermic reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents